The chemical industry is a powerhouse of innovation, driving advancements in fields as diverse as pharmaceuticals, materials science, and environmental engineering. Among the myriad substances that chemists work with, Tempo free radical stands out as a critical reagent with broad applications, especially in organic synthesis and polymer chemistry. Its unique properties enable chemists to catalyze reactions and create products that are otherwise challenging to produce. However, as with any powerful chemical, Tempo free radical presents significant risks that must be carefully managed to ensure the safety of laboratory personnel.
Chemical Properties of Tempo Free Radical
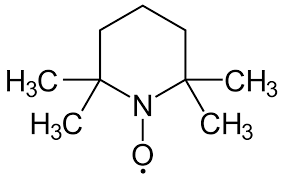
Structural Composition of Tempo
Tempo, short for 2,2,6,6-tetramethylpiperidine-1-oxyl, is a stable nitroxyl radical. Its structure consists of a piperidine ring with a nitroxyl group (NO•) attached, and the stability of this radical is bolstered by the electron-donating effects of the methyl groups. This unique configuration makes Tempo an excellent mediator in oxidation reactions, particularly in the conversion of alcohols to aldehydes or ketones.
Reactivity and Stability
Despite its radical nature, Tempo is remarkably stable, a characteristic that underpins its widespread use in organic chemistry. It is resistant to decomposition under ambient conditions and can be stored for extended periods without significant degradation. However, its stability does not negate its reactivity; Tempo is highly reactive in the presence of other radicals or oxidizing agents, which requires chemists to handle it with care.
Health Hazards Associated with Tempo Free Radical
Toxicity Levels
Tempo free radical is not without its hazards. While it is less toxic than many other free radicals, it still poses a significant risk, particularly if inhaled or absorbed through the skin. Prolonged exposure can lead to adverse health effects, including respiratory distress and skin irritation.
Routes of Exposure
Chemists are most at risk of exposure to Tempo through inhalation, skin contact, and accidental ingestion. The most common route is inhalation, where vapors or dust particles can enter the respiratory system, potentially causing acute or chronic health issues.
Symptoms of Overexposure
Symptoms of overexposure to Tempo can vary depending on the level and duration of exposure. Short-term exposure may cause irritation of the eyes, skin, and respiratory tract, while long-term exposure could lead to more severe conditions such as respiratory difficulties, headaches, and dizziness. In extreme cases, prolonged contact may cause organ damage, underscoring the need for rigorous safety protocols.
Safety Precautions for Handling Tempo Free Radical
Personal Protective Equipment (PPE)
When working with Tempo free radical, personal protective equipment (PPE) is non-negotiable. The selection of appropriate PPE is crucial to prevent exposure and ensure the safety of laboratory personnel. Chemists should wear gloves, lab coats, and safety goggles as a minimum standard when handling Tempo. For more extensive protection, especially in situations where exposure risk is high, face shields, respiratory protection, and chemical-resistant aprons should also be considered.
Safe Storage Practices
Tempo should be stored in tightly sealed containers in a cool, dry, and well-ventilated area, away from incompatible substances such as strong acids, bases, and oxidizing agents. The storage area should be clearly labeled and restricted to trained personnel to minimize the risk of accidental exposure.
Controlled Environment Requirements
The handling of Tempo should ideally occur in a fume hood or another controlled environment to prevent the escape of vapors or dust into the laboratory atmosphere. This controlled environment helps maintain air quality and reduces the risk of inhalation exposure for chemists.
Safe Storage Guidelines for Tempo Free Radical
Temperature Control
Maintaining the correct storage temperature for Tempo is crucial for preserving its stability and reducing the risk of accidental decomposition. It should be stored at room temperature, but not in direct sunlight or near heat sources, as elevated temperatures could destabilize the compound.
Proper Labeling and Containment
Proper labeling is essential for all hazardous chemicals, and Tempo is no exception. Containers should be clearly marked with the chemical name, concentration, and hazard warnings. Secondary containment, such as chemical storage cabinets, can provide an additional layer of protection against leaks or spills.
Minimizing Contamination Risks
Cross-contamination is a significant risk when working with reactive chemicals like Tempo. To minimize this risk, chemists should ensure that containers are tightly sealed after use and that equipment used in handling Tempo is thoroughly cleaned or designated solely for this purpose.
Personal Protective Equipment (PPE) for Tempo Handling
Types of Gloves for Chemical Safety
Not all gloves provide adequate protection against Tempo free radical. Chemists should use gloves made from materials that are resistant to organic solvents and radicals, such as nitrile or neoprene. These materials offer better resistance to chemical penetration than standard latex gloves.
Eye and Face Protection
Safety goggles are essential when handling Tempo to protect against splashes and accidental contact. In environments where there is a risk of significant exposure, a full-face shield may be warranted to provide comprehensive facial protection. triflic acid manufacturers is produced by several leading chemical manufacturers known for their expertise in specialty chemicals.
Laboratory Coats and Aprons
A lab coat made from flame-resistant and chemical-resistant material should be worn to protect against splashes and spills. In more hazardous situations, a chemical-resistant apron may also be necessary to protect the body from exposure.
Proper Disposal of Tempo Free Radical
Disposal Methods
Proper disposal of Tempo free radical is crucial to prevent environmental contamination and ensure laboratory safety. It should never be disposed of down the drain or in regular trash. Instead, Tempo waste should be collected in designated hazardous waste containers and disposed of following the laboratory’s hazardous waste management procedures.
Environmental Considerations
Due to its reactive nature, Tempo can pose environmental risks if not disposed of properly. It is essential to follow all regulatory guidelines for hazardous waste disposal to minimize environmental impact. Chemists should also consider waste reduction strategies, such as minimizing the amount of Tempo used in reactions, to reduce the volume of hazardous waste generated.
Emergency Response for Tempo Exposure
Immediate Actions in Case of Exposure
In the event of exposure to Tempo free radical, immediate action is critical to minimize harm. If skin contact occurs, the affected area should be flushed with water for at least 15 minutes, and contaminated clothing should be removed. If Tempo is inhaled, the exposed individual should be moved to fresh air immediately.
First Aid Measures
Basic first aid measures include rinsing the eyes with water if Tempo comes into contact with them, and seeking medical attention for significant exposures. It is important for laboratory personnel to be trained in first aid and to have access to emergency equipment such as eye wash stations and safety showers.